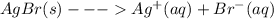Equilibrium shifts for slightly soluble compounds the reaction for the formation of a saturated solution of silver bromide (agbr) can be represented as agbr(s)? ag+(aq)+br? (aq)
consider that this reaction is an endothermic process and that the agbr solution is saturated.
classify the following changes based on whether they will favor the formation of reactant, whether they will favor the formation of products, or whether they will have no effect on the equilibrium.
1. adding hydrobromic acid (hbr)
2. lowering the concentration of bromide (br-) ions
3. heating the concentrations
4. adding solid silver bromide (agbr)
5. removing silver (ag+) ions

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Equilibrium shifts for slightly soluble compounds the reaction for the formation of a saturated solu...
Questions

Mathematics, 29.06.2019 14:50

Mathematics, 29.06.2019 14:50

Mathematics, 29.06.2019 14:50

Chemistry, 29.06.2019 14:50



Chemistry, 29.06.2019 14:50

Chemistry, 29.06.2019 14:50




Chemistry, 29.06.2019 14:50





Computers and Technology, 29.06.2019 14:50