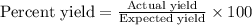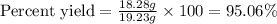Chemistry, 07.11.2019 07:31 ZaNiyahlove4711
Calculate the % yield if the amount of alum obtained was 18.28 g and the expected amount was 19.93 g.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Calculate the % yield if the amount of alum obtained was 18.28 g and the expected amount was 19.93 g...
Questions

Mathematics, 25.11.2019 03:31


Mathematics, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31




Mathematics, 25.11.2019 03:31

Physics, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31

English, 25.11.2019 03:31

Mathematics, 25.11.2019 03:31

History, 25.11.2019 03:31