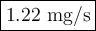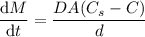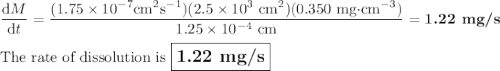Chemistry, 07.11.2019 21:31 alivas6618
Calculate the rate of dissolution (dm/dt) of relatively hydrophobic drug particles with a surface area of 2.5×103 cm2 and saturation solubility of 0.35 mg/ml at 25°c in water. the diffusion coefficient is 1.75×10-7 cm2/s, and the thickness of the diffusion layer is 1.25m. the concentration of the drug in the bulk solution is 2.1×10-4 mg/ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3


Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
Calculate the rate of dissolution (dm/dt) of relatively hydrophobic drug particles with a surface ar...
Questions

History, 27.02.2020 17:01

Computers and Technology, 27.02.2020 17:01





Mathematics, 27.02.2020 17:02


Computers and Technology, 27.02.2020 17:02

Computers and Technology, 27.02.2020 17:02



Mathematics, 27.02.2020 17:03



Computers and Technology, 27.02.2020 17:03



Computers and Technology, 27.02.2020 17:03