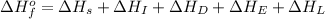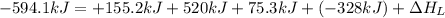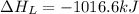Chemistry, 07.11.2019 22:31 kraigstlistt
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is called the born-haber cycle, which is a series of thermochemical processes, each with a δh, that add up (think of hess’s law) to complete a 5 step process for the formation of the salt. given the following data, calculate the lattice energy per mole of lif(s) formed. li(s) → li(g) δh°

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is calle...
Questions




Mathematics, 21.04.2020 15:31











English, 21.04.2020 15:31


Mathematics, 21.04.2020 15:31



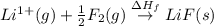
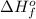 = enthalpy of formation of lithium fluoride = -594.1 kJ
= enthalpy of formation of lithium fluoride = -594.1 kJ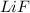 :
: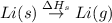
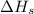 = sublimation energy of lithium = +155.2 kJ
= sublimation energy of lithium = +155.2 kJ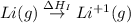
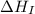 = ionization energy of lithium = +520 kJ
= ionization energy of lithium = +520 kJ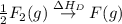
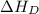 = dissociation energy of fluorine = +75.3 kJ
= dissociation energy of fluorine = +75.3 kJ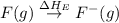
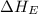 = electron affinity energy of fluorine = -328 kJ
= electron affinity energy of fluorine = -328 kJ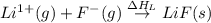
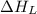 = lattice energy of lithium fluoride = ?
= lattice energy of lithium fluoride = ?