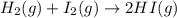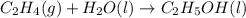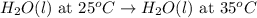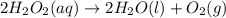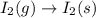Chemistry, 08.11.2019 00:31 salmanderabdi12
Determine whether the entropy, δs, increases or decreases for the following reactions. in some cases, more information is needed. mark those as "more information needed". h2 (g) + i2 (g) → 2hi (g). c2h4 (g) + h2o (l) → c2h5oh (l) h2o (l) at 25oc → h2o (l) at 35 oc 2h2o2 (aq) → 2 h2o (l) + o2 (g) i2 (g) → i2 (s)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1
You know the right answer?
Determine whether the entropy, δs, increases or decreases for the following reactions. in some cases...
Questions

Spanish, 08.04.2021 22:20

English, 08.04.2021 22:20

History, 08.04.2021 22:20




Mathematics, 08.04.2021 22:20


Mathematics, 08.04.2021 22:20






English, 08.04.2021 22:20

Computers and Technology, 08.04.2021 22:20




Mathematics, 08.04.2021 22:20