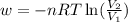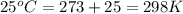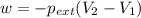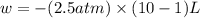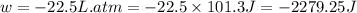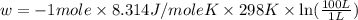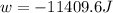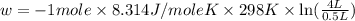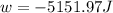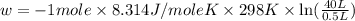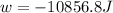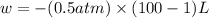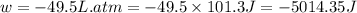Order the follow processes from (1) the least work done by the system to (5) the most work done by one mole of an ideal gas at 25°c. 1. an isothermal expansion from 1 l to 10 l at an external pressure of 2.5 atm. 2. a free isothermal expansion from 1 l to 100 l. 3. a reversible isothermal expansion from 0.5 l to 4 l. 4. a reversible isothermal expansion from 0.5 l to 40 l. 5. an isothermal expansion from 1 l to 100 l at an external pressure of 0.5 atm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Order the follow processes from (1) the least work done by the system to (5) the most work done by o...
Questions

Mathematics, 22.04.2021 20:10

English, 22.04.2021 20:10


Mathematics, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10

History, 22.04.2021 20:10


Geography, 22.04.2021 20:10




Chemistry, 22.04.2021 20:10


Mathematics, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10


History, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10

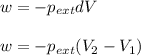
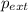 = external pressure
= external pressure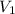 = initial volume of gas
= initial volume of gas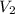 = final volume of gas
= final volume of gas