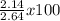Chemistry, 08.11.2019 01:31 jennychrin95
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: cao (s) + h 2o (l) → ca(oh) 2 (s) in a particular experiment, a 2.00-g sample of cao is reacted with excess water and 2.14 g of ca(oh) 2 is recovered. what is the percent yield in this experiment?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: cao (s) + h...
Questions

Mathematics, 06.06.2020 20:59




Mathematics, 06.06.2020 20:59


Biology, 06.06.2020 20:59

History, 06.06.2020 20:59

Mathematics, 06.06.2020 20:59



English, 06.06.2020 20:59






Computers and Technology, 06.06.2020 20:59


Mathematics, 06.06.2020 20:59