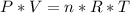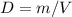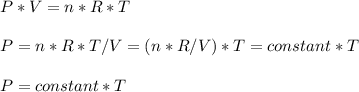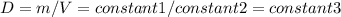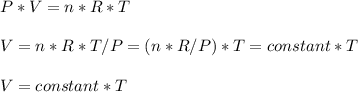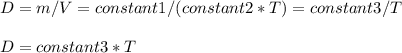Chemistry, 08.11.2019 03:31 kamkam5791
Consider two different containers, each filled with of . one of the containers is rigid and has constant volume. the other container is flexible (like a balloon) and is capable of changing its volume to keep the external pressure and internal pressure equal to each other. if you raise the temperature in both containers, what happens to the pressure and density of the gas inside each container? assume a constant external pressure.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Consider two different containers, each filled with of . one of the containers is rigid and has cons...
Questions


Chemistry, 03.02.2021 22:20


Computers and Technology, 03.02.2021 22:20



Computers and Technology, 03.02.2021 22:20

Mathematics, 03.02.2021 22:20

Health, 03.02.2021 22:20

Mathematics, 03.02.2021 22:20

Mathematics, 03.02.2021 22:20


History, 03.02.2021 22:20

Advanced Placement (AP), 03.02.2021 22:20


Physics, 03.02.2021 22:20




English, 03.02.2021 22:20