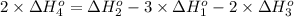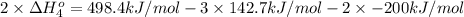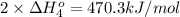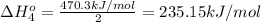Chemistry, 08.11.2019 07:31 destinystanley3794
Calculate the standard reaction enthalpy for the reaction no2(g) → no(g) + o(g) given +142.7 kj/mol for the standard enthalpy of formation of ozone and o2(g) → 2 o(g) ∆h ◦ = +498.4 kj/mol no(g) + o3(g) → no2(g) + o2(g) ∆h◦ = −200 kj/mol remember the definition of the standard enthalpy of formation of a substance.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Calculate the standard reaction enthalpy for the reaction no2(g) → no(g) + o(g) given +142.7 kj/mol...
Questions



Social Studies, 15.01.2020 18:31

Geography, 15.01.2020 18:31

Chemistry, 15.01.2020 18:31

Health, 15.01.2020 18:31



Mathematics, 15.01.2020 18:31

Social Studies, 15.01.2020 18:31

English, 15.01.2020 18:31

History, 15.01.2020 18:31

Mathematics, 15.01.2020 18:31

Mathematics, 15.01.2020 18:31



Mathematics, 15.01.2020 18:31


Computers and Technology, 15.01.2020 18:31

Biology, 15.01.2020 18:31

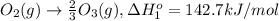 ..[1]
..[1]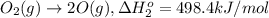 ..[2]
..[2]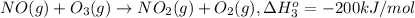 ..[3]
..[3]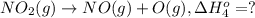 ..[4]
..[4]