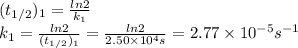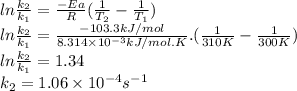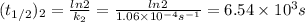For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k...

Chemistry, 08.11.2019 22:31 alexciamartinez05
For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kj/mol. what is the half-life at 310 k? (hint: use rate law expression to determine the reaction order → solve for k1 at 300 k using the corresponding half-life expression → use two-point arrhenius equation to solve for k2 at 310 k → use the half-life expression again to solve for half-life at 310 k)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
Questions



English, 12.05.2021 09:50

History, 12.05.2021 09:50




Chemistry, 12.05.2021 09:50


Mathematics, 12.05.2021 09:50

Mathematics, 12.05.2021 09:50

Mathematics, 12.05.2021 09:50



English, 12.05.2021 09:50


Social Studies, 12.05.2021 09:50

Mathematics, 12.05.2021 09:50


Mathematics, 12.05.2021 09:50

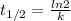
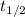 is the half-life
is the half-life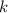 is the rate constant
is the rate constant