
Chemistry, 08.11.2019 23:31 astultz309459
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a thermos flask. when the reaction is complete, the temperature is 27.8°c. assuming that the solutions have the same heat capacity as pure water, compute the heat released (in kj).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1


Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a...
Questions


Computers and Technology, 21.05.2020 01:58



Social Studies, 21.05.2020 01:58



Biology, 21.05.2020 01:58

Mathematics, 21.05.2020 01:58









Geography, 21.05.2020 01:58



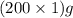 = 200 g
= 200 g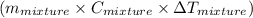
 represents change in temperature
represents change in temperature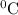 )
)![[400g\times 4.186J.g^{-1}.^{0}\textrm{C}^{-1}\times(27.8-21.0)^{0}\textrm{C} ]](/tpl/images/0366/2490/9c7ad.png) =
= 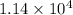 J=11.4 kJ
J=11.4 kJ

