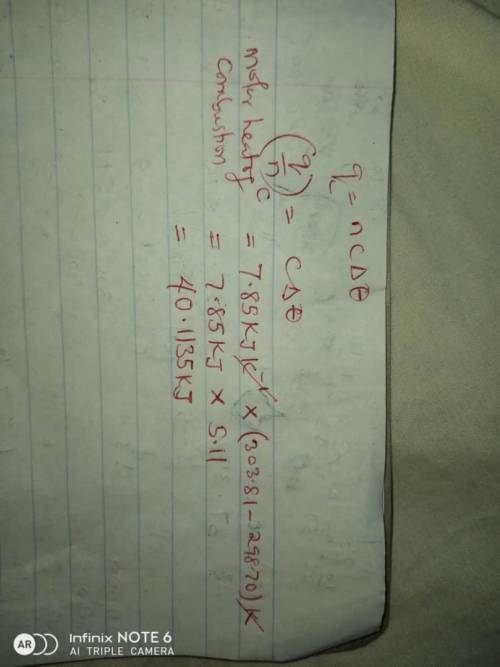Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3


You know the right answer?
A1.11 g sample of caffeine, c8h10n4o2, burns in a constant-volume calorimeter that has a heat capaci...
Questions

Mathematics, 16.01.2020 23:31


Mathematics, 16.01.2020 23:31

Mathematics, 16.01.2020 23:31



Chemistry, 16.01.2020 23:31


Physics, 16.01.2020 23:31


Spanish, 16.01.2020 23:31



History, 16.01.2020 23:31

Chemistry, 16.01.2020 23:31


Mathematics, 16.01.2020 23:31



Mathematics, 16.01.2020 23:31