
Chemistry, 09.11.2019 00:31 haydencheramie
Imagine that you are in chemistry lab and need to make 1.00 l of a solution with a ph of 2.80. you have in front of you 100 ml of 7.00×10−2 m hcl, 100 ml of 5.00×10−2 m naoh, and plenty of distilled water. you start to add hcl to a beaker of water when someone asks you a question. when you return to your dilution, you accidentally grab the wrong cylinder and add some naoh. once you realize your error, you assess the situation. you have 84.0 ml of hcl and 88.0 ml of naoh left in their original containers. part a assuming the final solution will be diluted to 1.00 l , how much more hcl should you add to achieve the desired ph?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
Imagine that you are in chemistry lab and need to make 1.00 l of a solution with a ph of 2.80. you h...
Questions



Advanced Placement (AP), 04.05.2021 08:20


Physics, 04.05.2021 08:20


English, 04.05.2021 08:20




History, 04.05.2021 08:20

History, 04.05.2021 08:20

English, 04.05.2021 08:20

Mathematics, 04.05.2021 08:20


Biology, 04.05.2021 08:20



English, 04.05.2021 08:20

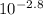 = [H⁺][H⁺] = 1.58 x 10⁻³ MThen we use the definition of Molarity [C=n/V]:[H⁺] = 1.58 x 10⁻³ M = molesH⁺ / 1.00 LmolesH⁺ = 1.58 x 10⁻³
= [H⁺][H⁺] = 1.58 x 10⁻³ MThen we use the definition of Molarity [C=n/V]:[H⁺] = 1.58 x 10⁻³ M = molesH⁺ / 1.00 LmolesH⁺ = 1.58 x 10⁻³

