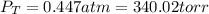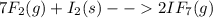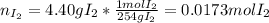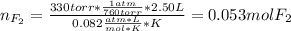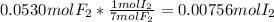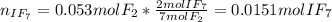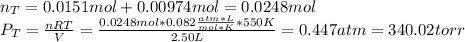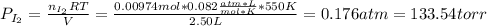Chemistry, 09.11.2019 05:31 jimennacastillo15
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine heptafluoride forms.3.30 × 102 torr of f2 and 4.40 g of solid i2 are put into a 2.50 l container at 2.50 × 102 k and the container is heated to 5.50 × 10^2 k.
(a) what is the final pressure?
ptotal =
(b) what is the partial pressure of i2 gas?
pi2 = i2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine hep...
Questions


History, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01


History, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01


English, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Computers and Technology, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01