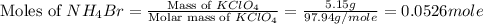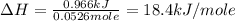When a solid dissolves in water, heat may be evolved or absorbed. the heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. in the laboratory a general chemistry student finds that when 5.15 g of nh4br(s) are dissolved in 105.00 g of water, the temperature of the solution drops from 23.82 to 21.63 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.76 j/°c. based on the student's observation, calculate the enthalpy of dissolution of nh4br(s) in kj/mol.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

You know the right answer?
When a solid dissolves in water, heat may be evolved or absorbed. the heat of dissolution (dissolvin...
Questions



Mathematics, 17.11.2020 21:40


Mathematics, 17.11.2020 21:40

Mathematics, 17.11.2020 21:40





Health, 17.11.2020 21:40


English, 17.11.2020 21:40




Mathematics, 17.11.2020 21:40

English, 17.11.2020 21:40

Health, 17.11.2020 21:40

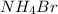 is 18.4 kJ/mole
is 18.4 kJ/mole![q=[q_1+q_2]](/tpl/images/0366/8017/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0366/8017/1d50b.png)
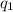 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter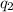 = heat absorbed by the water
= heat absorbed by the water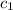 = specific heat of calorimeter =
= specific heat of calorimeter = 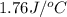
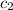 = specific heat of water =
= specific heat of water = 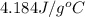
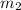 = mass of water = 105.00 g
= mass of water = 105.00 g = change in temperature =
= change in temperature = 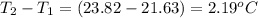
![q=[(1.76J/^oC\times 2.19^oC)+(105.00g\times 4.184J/g^oC\times 2.19^oC)]](/tpl/images/0366/8017/9228d.png)
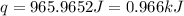
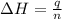
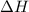 = enthalpy change = ?
= enthalpy change = ?