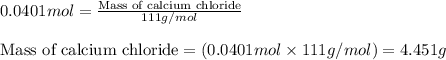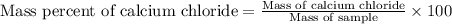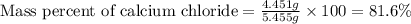Chemistry, 09.11.2019 08:31 Caixiayang3613
1. a 5.455-g sample of impure cacl2 is dissolved and treated with excess potassium carbonate solution. the dried caco3 (calcium carbonate) precipitate weighs 4.010-g. calculate the percent by mass of cacl2 in the original mixture.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1


Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
1. a 5.455-g sample of impure cacl2 is dissolved and treated with excess potassium carbonate solutio...
Questions

Chemistry, 04.11.2020 18:40

History, 04.11.2020 18:40

Physics, 04.11.2020 18:40






Mathematics, 04.11.2020 18:40


Chemistry, 04.11.2020 18:40

Arts, 04.11.2020 18:40

Law, 04.11.2020 18:40

Physics, 04.11.2020 18:40

English, 04.11.2020 18:40


Biology, 04.11.2020 18:40

Computers and Technology, 04.11.2020 18:40


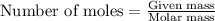 .....(1)
.....(1)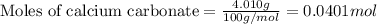
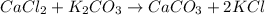
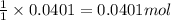 of calcium chloride
of calcium chloride