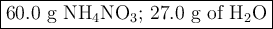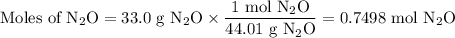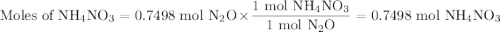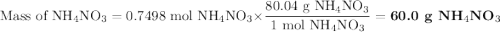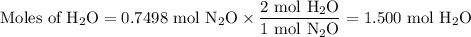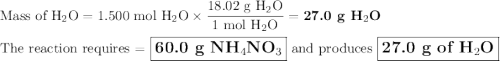Piu
1. laughing gas in
produced wh
shing gas (nitrous oxide, n, o) is sometimes us...

Chemistry, 09.11.2019 15:31 ahankaranth
Piu
1. laughing gas in
produced wh
shing gas (nitrous oxide, n, o) is sometimes used as an anesthetic in dentistry. it is
duced when ammonium nitrate is decomposed according to the following reaction.
nh, no3(s) n2o(g) + 2h, o(1)
how many grams of nh no, are required to produce 33.0 g n, o?
how many grams of water are produced in this reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Questions

Mathematics, 23.08.2019 15:50




Spanish, 23.08.2019 15:50



Computers and Technology, 23.08.2019 15:50


Chemistry, 23.08.2019 15:50

Mathematics, 23.08.2019 15:50


Mathematics, 23.08.2019 15:50


Biology, 23.08.2019 15:50


Mathematics, 23.08.2019 15:50

Social Studies, 23.08.2019 15:50

Mathematics, 23.08.2019 15:50