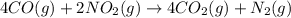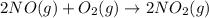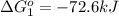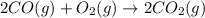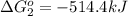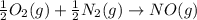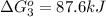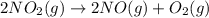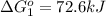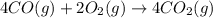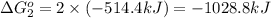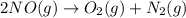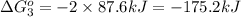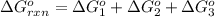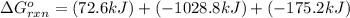Chemistry, 10.11.2019 01:31 TheOriginal2x
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reactions and given δg∘rxn values: a) 2no(g)+o2(g)→2no2(g), δg∘rxn= - 72.6 kjb) 2co(g)+o2(g)→2co2(g), δg∘rxn= - 514.4 kjc) 12o2(g)+12n2(g)→no(g), δg∘rxn= 87.6 kj

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


You know the right answer?
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reaction...
Questions









Mathematics, 24.11.2021 14:00

Social Studies, 24.11.2021 14:00


Social Studies, 24.11.2021 14:00

History, 24.11.2021 14:00

Computers and Technology, 24.11.2021 14:00

English, 24.11.2021 14:00



Computers and Technology, 24.11.2021 14:00


Mathematics, 24.11.2021 14:00

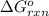 for the reaction is -1131.4 kJ
for the reaction is -1131.4 kJ