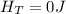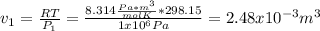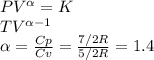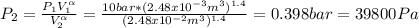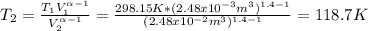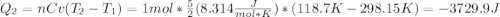Chemistry, 10.11.2019 02:31 thegent1859
One mole of an ideal gas in a closed system, initially at 25°c and 10 bar, is first expanded adiabatically, then heated isochorically to reach a final state of 25°c and 1 bar. assuming these processes are mechanically reversible, compute t and p after the adiabatic expansion, and compute q, w, δu, and δh for each step and for the overall process. take cp = (7/2)r and cv = (5/2)r.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
One mole of an ideal gas in a closed system, initially at 25°c and 10 bar, is first expanded adiabat...
Questions

Business, 22.06.2019 08:00

Biology, 22.06.2019 08:00




Physics, 22.06.2019 08:00

Spanish, 22.06.2019 08:00


Mathematics, 22.06.2019 08:10






Mathematics, 22.06.2019 08:10





Arts, 22.06.2019 08:10