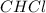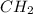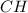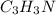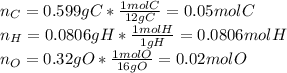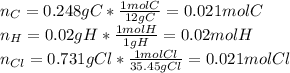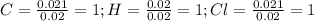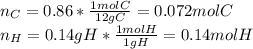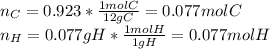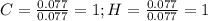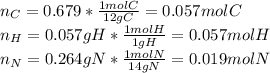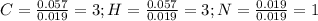Polymers are large molecules composed of simple units repeated many times. thus, they often have relatively simple empirical formulas. calculate the empirical formulas of the following polymers: (a) lucite (plexiglas); 59.9% c, 8.06% h, 32.0% o (b) saran; 24.8% c, 2.0% h, 73.1% cl (c) polyethylene; 86% c, 14% h (d) polystyrene; 92.3% c, 7.7% h (e) orlon; 67.9% c, 5.70% h, 26.4% n

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Polymers are large molecules composed of simple units repeated many times. thus, they often have rel...
Questions




Physics, 08.12.2019 07:31


Mathematics, 08.12.2019 07:31



History, 08.12.2019 07:31

Mathematics, 08.12.2019 07:31





English, 08.12.2019 07:31



Mathematics, 08.12.2019 07:31

Social Studies, 08.12.2019 07:31