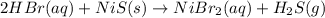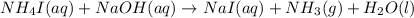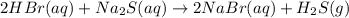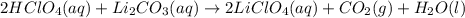Chemistry, 10.11.2019 02:31 mikehager4321
59. complete and balance each gas-evolution equation. missed this? read section 5.8; watch kcv 5.5 a. hbr(aq) + nis(s) ¡ b. nh4i(aq) + naoh(aq) ¡ c. hbr(aq) + na2s(aq) ¡ d. hclo4(aq) + li2co3(aq) ¡ tro, nivaldo j.. chemistry (p. 206). pearson education. kindle edition.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
59. complete and balance each gas-evolution equation. missed this? read section 5.8; watch kcv 5.5...
Questions








English, 10.10.2019 01:30



Mathematics, 10.10.2019 01:30


Mathematics, 10.10.2019 01:30



History, 10.10.2019 01:30



Health, 10.10.2019 01:30