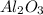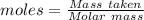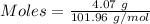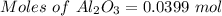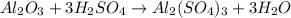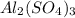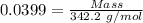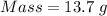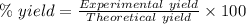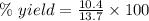Chemistry, 10.11.2019 03:31 babyboogrocks5572
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the reaction yields 10.4 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) what is the theoretical yield of aluminum sulfate? grams what is the percent yield for this reaction? %

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the re...
Questions

Mathematics, 15.10.2019 18:00










Computers and Technology, 15.10.2019 18:00




Mathematics, 15.10.2019 18:00

Mathematics, 15.10.2019 18:00

Mathematics, 15.10.2019 18:00


Mathematics, 15.10.2019 18:00