
Chemistry, 10.11.2019 03:31 chantianabess36
You're provided with a bottle labled [cocl2.6h2o] = 0.051 m in 4.60 m hcl. you heat a small volume of the solution in a hot water bath to 50 ∘c. if you determine that [cocl42-] = 0.031 m at 50 ∘c at equilibrium, what must be the equilibrium concentration of [cl-] at 50 ∘c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
You're provided with a bottle labled [cocl2.6h2o] = 0.051 m in 4.60 m hcl. you heat a small volume o...
Questions

History, 18.11.2019 06:31

Mathematics, 18.11.2019 06:31



Social Studies, 18.11.2019 06:31


History, 18.11.2019 06:31



History, 18.11.2019 06:31

Mathematics, 18.11.2019 06:31


Mathematics, 18.11.2019 06:31



Mathematics, 18.11.2019 06:31


History, 18.11.2019 06:31

History, 18.11.2019 06:31

Mathematics, 18.11.2019 06:31

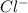 at equilibrium is 4.54 M
at equilibrium is 4.54 M![[CoCl_2.6H_2O]+2Cl^-\rightleftharpoons [CoCl_4]^{2-}+6H_2O](/tpl/images/0367/5714/8c100.png)
![[CoCl_4]^{2-}](/tpl/images/0367/5714/84955.png) at equilibrium = 0.031 M
at equilibrium = 0.031 M

