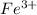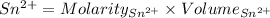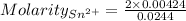Chemistry, 10.11.2019 04:31 xxtonixwilsonxx
Awire weighing 0.250 g and containing 94.75% fe is dissolved in hcl. the iron is completely oxidized to fe3+ by bromine water. the solution is then treated with tin(ii) chloride to bring about the reaction sn2+(aq) + 2fe3+(aq) → 2fe2+(aq) + sn4+(aq) + h2o(l) if 24.4 ml of tin(ii) chloride solution is required for complete reaction, what is the molarity of the tin(ii) chloride solution

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
You know the right answer?
Awire weighing 0.250 g and containing 94.75% fe is dissolved in hcl. the iron is completely oxidized...
Questions


Mathematics, 03.03.2020 20:47






Mathematics, 03.03.2020 20:47

Mathematics, 03.03.2020 20:47



Mathematics, 03.03.2020 20:47

Mathematics, 03.03.2020 20:47




English, 03.03.2020 20:47

Mathematics, 03.03.2020 20:47


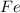 is 94.75 % by mass.
is 94.75 % by mass.
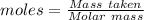
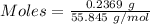
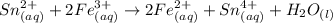
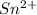 = 2*Moles of
= 2*Moles of