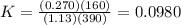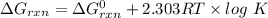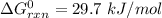Chemistry, 10.11.2019 05:31 elijahjwhite15
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentrations, calculate the free energy change for this reaction at 37.0 °c (310 k). δg°\' for the reaction is 29.7 kj/mol. assume that the reaction occurs at ph 7. [malate] = 1.13 mm [oxaloacetate] = 0.270 mm [nad ] = 390 mm [nadh] = 160 mm

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentra...
Questions



Geography, 22.11.2019 20:31


History, 22.11.2019 20:31


Law, 22.11.2019 20:31



Mathematics, 22.11.2019 20:31









Mathematics, 22.11.2019 20:31

Chemistry, 22.11.2019 20:31


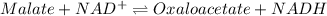
![K =\frac {[oxaloacetate][NADH]}{[malate][NAD^+]}](/tpl/images/0367/7081/e135a.png)
![[NAD^+]](/tpl/images/0367/7081/f5e9f.png) = 390 mM
= 390 mM