
Chemistry, 10.11.2019 05:31 battlemarshmell
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . if of sodium sulfate is produced from the reaction of of sulfuric acid and of sodium hydroxide, calculate the percent yield of sodium sulfate. be sure your answer has the correct number of significant digits in it.

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liqui...
Questions









Mathematics, 16.10.2019 22:00



Chemistry, 16.10.2019 22:00




Social Studies, 16.10.2019 22:00


Mathematics, 16.10.2019 22:00


History, 16.10.2019 22:00


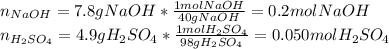
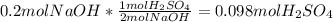

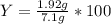
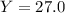 %
%

