
Chemistry, 10.11.2019 05:31 jalenclarke25
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2co3) and the bicarbonate (hydrogen carbonate, hco3-) ion. what is the ratio of [bicarbonate]/[carbonic acid] at this ph? for carbonic acid, ka1 = 4.2 10-7.a) [bicarbonae]/[carbonic acid] = 0.11.b) [bicarbonate]/[carbonic acid] = 9.4.c) [bicarbonate]/[carbonic acid] = 0.38.d) none of the above ratios is correct. e) [bicarbonate]/[carbonic acid] = 2.65.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1


Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2...
Questions

Geography, 26.09.2019 13:00

Mathematics, 26.09.2019 13:00



English, 26.09.2019 13:00

Biology, 26.09.2019 13:00

Social Studies, 26.09.2019 13:00

Physics, 26.09.2019 13:00




Mathematics, 26.09.2019 13:00

Social Studies, 26.09.2019 13:00


Mathematics, 26.09.2019 13:00

Physics, 26.09.2019 13:00

Social Studies, 26.09.2019 13:00

Mathematics, 26.09.2019 13:10

Mathematics, 26.09.2019 13:10

Mathematics, 26.09.2019 13:10

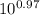 = [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4
= [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4


