Chemistry, 10.11.2019 06:31 Alienchild239
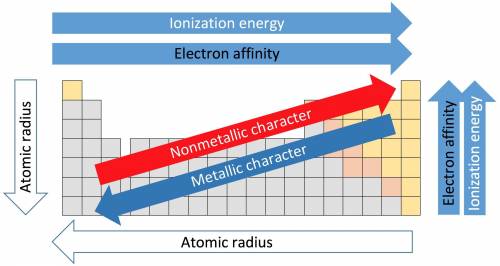
How do the periodic trends in metallic character compare to those for ionization energy? how do the periodic trends in metallic character compare to those for ionization energy? metals tend to have higher ionization energies than nonmetals. metals tend to have lower ionization energies than nonmetals. metals and nonmetals tend to have the same ionization energies.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
How do the periodic trends in metallic character compare to those for ionization energy? how do the...
Questions



Mathematics, 31.01.2022 23:00

Mathematics, 31.01.2022 23:00

Biology, 31.01.2022 23:00

Mathematics, 31.01.2022 23:00


Mathematics, 31.01.2022 23:00








Mathematics, 31.01.2022 23:00

Geography, 31.01.2022 23:10

Mathematics, 31.01.2022 23:10