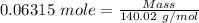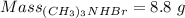Achemistry graduate student is given 250. ml of a 1.00 m trimethylamine cch, n solution. trimethylamine is a weak base with kb=7.4x 10 . what mass of (ch3)3nhbr should the student dissolve in the (ch3)3n solution to turn it into a buffer with ph-11.467 you may assume that the volume of the solution doesn't change when the (ch3) nhbr is dissolved in it. be sure your answer has a unit symbol, and round it to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Achemistry graduate student is given 250. ml of a 1.00 m trimethylamine cch, n solution. trimethylam...
Questions

Mathematics, 05.05.2020 05:20


Mathematics, 05.05.2020 05:20

Mathematics, 05.05.2020 05:20

Biology, 05.05.2020 05:20


Advanced Placement (AP), 05.05.2020 05:20





Mathematics, 05.05.2020 05:20

Social Studies, 05.05.2020 05:20

Business, 05.05.2020 05:20


History, 05.05.2020 05:20

Social Studies, 05.05.2020 05:20

Biology, 05.05.2020 05:20


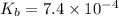
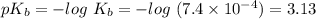
![ pOH=pK_b+log\frac{[salt]}{[base]} ](/tpl/images/0367/7618/73b50.png)
![ 2.533=3.13+log\frac{[salt]}{1.00} ](/tpl/images/0367/7618/759d7.png)

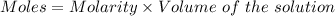
 = 0.2526*0.25 moles = 0.06315 moles
= 0.2526*0.25 moles = 0.06315 moles