
Chemistry, 10.11.2019 06:31 electrofy456
Calculate δh o rxn for the following: a. sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh o f [sio2 (s)] = −910.9 kj/mol δh o f [hf (g)] = −273 kj/mol δh o f [sif4 (g)] = −1,614.9 kj/mol δh o f [h2o (l)] = −285.840 kj/mol b. c2h6(g) + o2(g) co2(g) + h2o(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1



Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
Calculate δh o rxn for the following: a. sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh o f [sio2 (s)] =...
Questions


Social Studies, 26.05.2021 19:50





Mathematics, 26.05.2021 19:50



Mathematics, 26.05.2021 19:50

Mathematics, 26.05.2021 19:50



Mathematics, 26.05.2021 19:50

Social Studies, 26.05.2021 19:50

Mathematics, 26.05.2021 19:50

History, 26.05.2021 19:50

History, 26.05.2021 19:50

Mathematics, 26.05.2021 19:50

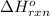 for the reaction is -183.68 kJ/mole
for the reaction is -183.68 kJ/mole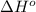
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0367/7523/45485.png)
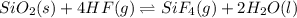
![\Delta H^o_{rxn}=[(n_{(SiF_4)}\times \Delta H^o_f_{(SiF_4)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(SiO_2)}\times \Delta H^o_f_{(SiO_2)})+(n_{(HF)}\times \Delta H^o_f_{(HF)})]](/tpl/images/0367/7523/cf6d4.png)
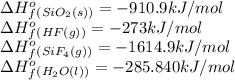
![\Delta H^o_{rxn}=[(1\times -1614.9)+(2\times -285.840)]-[(1\times -910.9)+(4\times -273)]=-183.68kJ/mol](/tpl/images/0367/7523/c5c6f.png)


