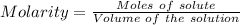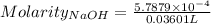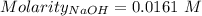Chemistry, 10.11.2019 06:31 maddietomlinson113
Ask your teacher sodium hydroxide solution is usually standardized by titrating a pure sample of potassium hydrogen phthalate (khc8h4o4, often abbreviated khp), an acid with one acidic hydrogen and a molar mass of 204.220 g/mol. it takes 36.01 ml of a sodium hydroxide solution to titrate a 0.1182-g sample of khp. what is the molarity of the sodium hydroxide

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Ask your teacher sodium hydroxide solution is usually standardized by titrating a pure sample of pot...
Questions

Mathematics, 25.03.2020 20:55





Mathematics, 25.03.2020 20:55



Mathematics, 25.03.2020 20:56



Mathematics, 25.03.2020 20:56


Mathematics, 25.03.2020 20:56



Mathematics, 25.03.2020 20:56

English, 25.03.2020 20:56

Biology, 25.03.2020 20:56

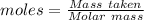
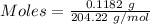
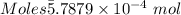
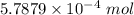 of KHP reacts with
of KHP reacts with