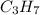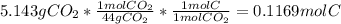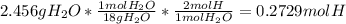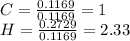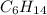A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5.143 g co2 (g) and 2.456 g h2o (l) on complete combustion. this particular compound is also found to be an alkane with one methyl group attached to a longer carbon chain and to have a molecular formula twice its empirical formula. the compound also has the following properties: melting point of -154 c , boiling point of 60.3 c , density of 0.6532 g/ml at 20 c , specific heat of 2.25 j/(g*c), and -204.6 kj/mol use the masses of carbon dioxide, co2, and water, h2o , to determine the empirical formula of the alkane component. express your answer as a chemical formula?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

You know the right answer?
A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5....
Questions






Mathematics, 28.07.2019 04:33


Mathematics, 28.07.2019 04:33

Mathematics, 28.07.2019 04:33



English, 28.07.2019 04:33


Mathematics, 28.07.2019 04:33





Computers and Technology, 28.07.2019 04:33

Health, 28.07.2019 04:33