Chemistry, 11.11.2019 21:31 pedroramirezr2
The "air bags" that are currently installed in automobiles to prevent injuries in the event of a crash are equipped with sodium azide, nan₃, which decomposes when activated by an electronic igniter to produce nitrogen gas that fills the bag. how many liters of nitrogen, measured at 25°c and 1.00 atm, will be produced by 100.0 g of nan₃?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
The "air bags" that are currently installed in automobiles to prevent injuries in the event of a cra...
Questions


Arts, 02.04.2021 18:40

Mathematics, 02.04.2021 18:40

Mathematics, 02.04.2021 18:40

Mathematics, 02.04.2021 18:40


Mathematics, 02.04.2021 18:40

History, 02.04.2021 18:40

Social Studies, 02.04.2021 18:40

Mathematics, 02.04.2021 18:40

Social Studies, 02.04.2021 18:40



Mathematics, 02.04.2021 18:40

English, 02.04.2021 18:40

Mathematics, 02.04.2021 18:40


History, 02.04.2021 18:40


Mathematics, 02.04.2021 18:40

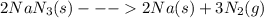
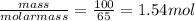
 mol
mol