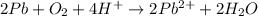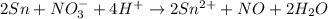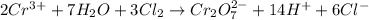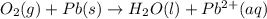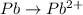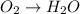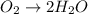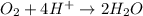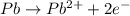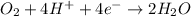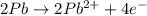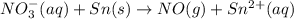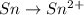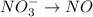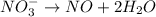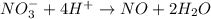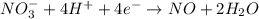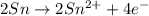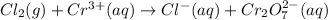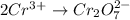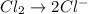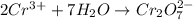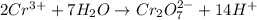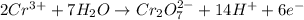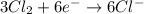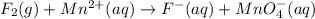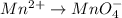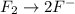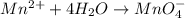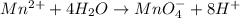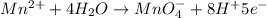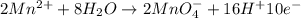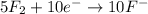Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-reaction method.
(use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.)
o2(g) + pb(s) → h2o(l) + pb2+(aq) (b) no3−(aq) + sn(s) → no(g) + sn2+(aq) (c) cl2(g) + cr3+(aq) → cl −(aq) + cr2o72−(aq) (d) f2(g) + mn2+(aq) → f −(aq) + mno4−(aq)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-...
Questions

Mathematics, 09.12.2020 08:10


Mathematics, 09.12.2020 08:10


Mathematics, 09.12.2020 08:10


English, 09.12.2020 08:10



History, 09.12.2020 08:10

Mathematics, 09.12.2020 08:10



Computers and Technology, 09.12.2020 08:10


Social Studies, 09.12.2020 08:10

Mathematics, 09.12.2020 08:10

Physics, 09.12.2020 08:10

Mathematics, 09.12.2020 08:10

Biology, 09.12.2020 08:10