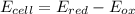Chemistry, 11.11.2019 23:31 mariamakonteh31
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are
cu2+(aq)+2e−→cu(s) and co(s)→co2+(aq)+2e−
the net reaction is
cu2+(aq)+co(s)→cu(s)+co2+(aq)
use the given standard reduction potentials in your calculation as appropriate. ( keq=5.88*10^20)
in the activity, click on the keq and δg∘ quantities to observe how they are related.
calculate δg∘ using this relationship and the equilibrium constant (keq) obtained in part a at t=298k: keq=5.88*10^20
express the gibbs free energy (δg∘) in joules to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2


Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 13:00
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
You know the right answer?
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this re...
Questions


Mathematics, 13.12.2019 05:31

Arts, 13.12.2019 05:31


Mathematics, 13.12.2019 05:31




English, 13.12.2019 05:31

Mathematics, 13.12.2019 05:31

Mathematics, 13.12.2019 05:31

English, 13.12.2019 05:31

English, 13.12.2019 05:31



Mathematics, 13.12.2019 05:31

Mathematics, 13.12.2019 05:31

Mathematics, 13.12.2019 05:31

Health, 13.12.2019 05:31