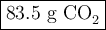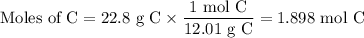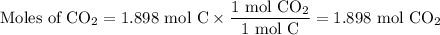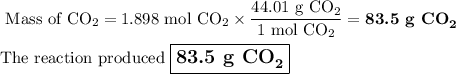Chemistry, 11.11.2019 23:31 jaylinthornton6
When 22.8 g of carbon were burned in the presence of 78. 9 g of oxygen 18.1 g of oxygen remained unreacted what mass of carbon dioxide was produced

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1



Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
When 22.8 g of carbon were burned in the presence of 78. 9 g of oxygen 18.1 g of oxygen remained unr...
Questions


Biology, 13.10.2020 01:01

History, 13.10.2020 01:01

History, 13.10.2020 01:01

Physics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01


Mathematics, 13.10.2020 01:01

Spanish, 13.10.2020 01:01


History, 13.10.2020 01:01

Chemistry, 13.10.2020 01:01

History, 13.10.2020 01:01


Mathematics, 13.10.2020 01:01


Mathematics, 13.10.2020 01:01