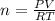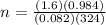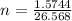Chemistry, 12.11.2019 04:31 ChloeLiz7111
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required to form 1.6 l of o2 at a temperature of 324 k and a pressure of 0.984 atm ?
express your answer using two significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
Questions


Biology, 19.08.2019 05:10


Physics, 19.08.2019 05:10



Mathematics, 19.08.2019 05:10

Geography, 19.08.2019 05:10


History, 19.08.2019 05:10





History, 19.08.2019 05:10

History, 19.08.2019 05:10

Social Studies, 19.08.2019 05:10

Physics, 19.08.2019 05:10

Geography, 19.08.2019 05:10

Physics, 19.08.2019 05:10