
250 milliliters of a concentrated iron (ii) ion solution are sitting on the lab bench in need of analysis to determine its exact concentration of fe2+ ions. as part of the analysis a 15 ml sample of this solution was diluted to a final volume of 100 ml. the iron (ii) ion concentration of the diluted solution was found to be 1.35 x 10-3 mg/ml fe2+. what was the total number of milligrams of iron (ii) that were contained in the original solution?
note that this question is not asking for how many mg/ml of iron (ii) where in the original solution, but the total mass of iron contained in the original solution. hint: you must first calculate the mg/ml of iron (ii) in the original solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

You know the right answer?
250 milliliters of a concentrated iron (ii) ion solution are sitting on the lab bench in need of ana...
Questions

Mathematics, 25.11.2020 03:30

Mathematics, 25.11.2020 03:30

Mathematics, 25.11.2020 03:30


English, 25.11.2020 03:30

History, 25.11.2020 03:30

Mathematics, 25.11.2020 03:30

Mathematics, 25.11.2020 03:30

Mathematics, 25.11.2020 03:30


Mathematics, 25.11.2020 03:30

Mathematics, 25.11.2020 03:30


Chemistry, 25.11.2020 03:30


Mathematics, 25.11.2020 03:30


Mathematics, 25.11.2020 03:30

Mathematics, 25.11.2020 03:30

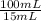 = 9,00x10⁻³ mg/mL
= 9,00x10⁻³ mg/mL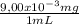 = 2,25 mg of Fe²⁺
= 2,25 mg of Fe²⁺

