
Chemistry, 12.11.2019 22:31 sydneykated
Write the reaction rate expressions for the reaction below in terms of the disappearance of the reactants and the appearance of products. give the expressions for the disappearance of the reactants first, in the order written in the chemical equation. then write the expressions for the appearance of the products in the order written in the chemical equation. write the expressions in order of appearance in the equation in the form. ± 1 x × δ[α] δt where ± is either a plus or a minus sign, not both, x is an integer, and a is a chemical species. do not include the state of matter. 2h2(g) + o2(g) → 2h2o(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2
You know the right answer?
Write the reaction rate expressions for the reaction below in terms of the disappearance of the reac...
Questions




Computers and Technology, 30.06.2020 17:01



English, 30.06.2020 17:01

English, 30.06.2020 17:01












Mathematics, 30.06.2020 17:01

![-\frac{1d[H_2]}{2dt}](/tpl/images/0371/2323/5d767.png) rate of disappearance of oxygen =
rate of disappearance of oxygen = ![-\frac{1d[O_2]}{1dt}](/tpl/images/0371/2323/77c77.png)
![+\frac{1d[H_2O]}{2dt}](/tpl/images/0371/2323/aa544.png)
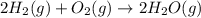
![Rate=k[H_2]^2[O_2]^1](/tpl/images/0371/2323/df759.png)


