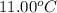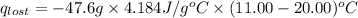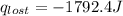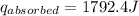A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water was 20.00°c. after the compound dissolved, the temperature of the water was 11.00°c. assume the heat was completely absorbed from the water and no heat was absorbed by the reaction container or the surroundings. calculate the heat absorbed by the process. the specific heat of water is 4.184 j/g·°c.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
You know the right answer?
A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water w...
Questions


History, 20.10.2020 23:01

History, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

English, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01


Biology, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01


History, 20.10.2020 23:01


Mathematics, 20.10.2020 23:01

Geography, 20.10.2020 23:01


History, 20.10.2020 23:01


Mathematics, 20.10.2020 23:01

Physics, 20.10.2020 23:01

Chemistry, 20.10.2020 23:01

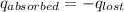
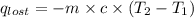
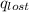 = heat lost by the water = ?
= heat lost by the water = ?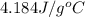
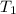 = initial temperature of water =
= initial temperature of water = 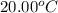
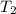 = final temperature of water =
= final temperature of water =