
Chemistry, 13.11.2019 00:31 staz13wiggins
The following sequence of reactions occurs in the commercial production of aqueous nitric acid. (the overall industrial process produces excess no.) 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(l) δh = −1166.0 kj/mol (1) 2 no(g) + o2(g) → 2 no2(g) δh = −116.2 kj/mol (2) 3 no2(g) + h2o(l) → 2 hno3(aq) + no(g) δh = −137.3 kj/mol (3) determine the total energy change (in kj) for the production of one mole of aqueous nitric acid by this process.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
The following sequence of reactions occurs in the commercial production of aqueous nitric acid. (the...
Questions


Mathematics, 03.02.2020 12:43

Mathematics, 03.02.2020 12:43

Social Studies, 03.02.2020 12:43

English, 03.02.2020 12:43


Health, 03.02.2020 12:43

English, 03.02.2020 12:43


English, 03.02.2020 12:43

English, 03.02.2020 12:43


Mathematics, 03.02.2020 12:43




Mathematics, 03.02.2020 12:43

History, 03.02.2020 12:43

Social Studies, 03.02.2020 12:43

Biology, 03.02.2020 12:43

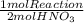 = 709,8 kJ
= 709,8 kJ

