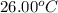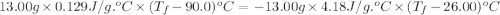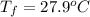Chemistry, 13.11.2019 00:31 amandamac7339
What is the final temperature of a system if 13.00 g of gold at 90.0°c is placed in 13.00 g of water at 26.00°c? the molar heat capacity of gold is 25.41 j/(mol · °c) and the heat capacity of water is 4.18 j/(g · °c).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
What is the final temperature of a system if 13.00 g of gold at 90.0°c is placed in 13.00 g of water...
Questions

English, 04.02.2020 20:57



History, 04.02.2020 20:57

Mathematics, 04.02.2020 20:57

Social Studies, 04.02.2020 20:57


Biology, 04.02.2020 20:57


Mathematics, 04.02.2020 20:57




Mathematics, 04.02.2020 20:58

Mathematics, 04.02.2020 20:58

Biology, 04.02.2020 20:58

History, 04.02.2020 20:58



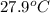
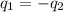
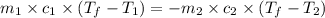
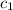 = specific heat of gold =
= specific heat of gold = 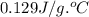
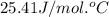
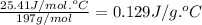
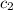 = heat capacity of water =
= heat capacity of water = 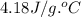
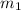 = mass of gold = 13.00 g
= mass of gold = 13.00 g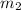 = mass of water = 13.00 g
= mass of water = 13.00 g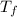 = final temperature of system = ?
= final temperature of system = ?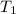 = initial temperature of gold =
= initial temperature of gold = 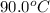
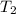 = initial temperature of water =
= initial temperature of water =