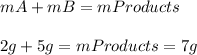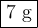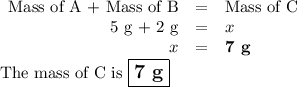Chemistry, 13.11.2019 00:31 monasiamcneill
the mass of chemical a is 2 g, and the mass of chemical b is 5 g. if the two chemicals are mixed and a
chemical reaction takes place, what is the mass of the end products?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
the mass of chemical a is 2 g, and the mass of chemical b is 5 g. if the two chemicals are mixed and...
Questions


Mathematics, 07.06.2020 18:57

Mathematics, 07.06.2020 18:57




Mathematics, 07.06.2020 18:57


Mathematics, 07.06.2020 18:57


English, 07.06.2020 18:57

Business, 07.06.2020 18:57

Social Studies, 07.06.2020 18:57


Mathematics, 07.06.2020 18:57


Mathematics, 07.06.2020 18:57

Mathematics, 07.06.2020 18:57


History, 07.06.2020 18:57