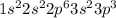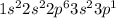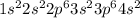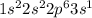Chemistry, 13.11.2019 01:31 serenityarts123
Select the correct statement below:
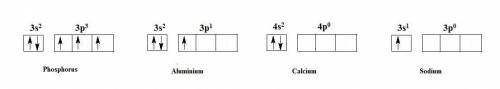
(a) phosphorous contains 10 core electrons and 5 valence electrons. its orbital diagram contains one half-filled 3p orbital and two filled 3p orbitals.
(b) aluminum contains 10 core electrons and 3 valence electrons. its orbital diagram contains three half-filled 3p orbitals.
(c) calcium contains 18 core electrons and 2 valence electrons. its orbital diagram contains two half-filled 4s orbitals and no filled 4p orbitals.
(d) sodium contains 10 core electrons and 1 valence electron. its orbital diagram contains one half-filled 3s orbital and three empty 3p orbitals.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1



Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Select the correct statement below:
(a) phosphorous contains 10 core electrons and 5 v...
(a) phosphorous contains 10 core electrons and 5 v...
Questions

Mathematics, 26.02.2021 02:50


Biology, 26.02.2021 02:50





Biology, 26.02.2021 02:50

Mathematics, 26.02.2021 02:50

Chemistry, 26.02.2021 02:50



Mathematics, 26.02.2021 02:50


Biology, 26.02.2021 02:50

English, 26.02.2021 02:50

Mathematics, 26.02.2021 02:50


Mathematics, 26.02.2021 03:00