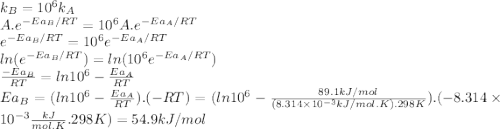Chemistry, 13.11.2019 02:31 joseperez1224
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k. reaction b is one million times faster than reaction a at the same temperature. the products of each reaction are 10.0 kj mol–1 (2.39 kcal mol–1) more stable than the reactants. (a) what is the standard free energy of activation of reaction b?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
The study of witch tree monkeys feed in is part of the science life
Answers: 1

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
The standard free energy of activation of a reaction a is 81.9 kj mol–1 (19.6 kcal mol–1) at 298 k....
Questions

Spanish, 16.11.2019 01:31

Social Studies, 16.11.2019 01:31


Health, 16.11.2019 01:31


Business, 16.11.2019 01:31

Spanish, 16.11.2019 01:31

Mathematics, 16.11.2019 01:31


Biology, 16.11.2019 01:31

Social Studies, 16.11.2019 01:31

Health, 16.11.2019 01:31

Mathematics, 16.11.2019 01:31



Physics, 16.11.2019 01:31

Mathematics, 16.11.2019 01:31

Mathematics, 16.11.2019 01:31


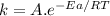
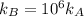 .
.