
Chemistry, 13.11.2019 06:31 PrincessKehlani15
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750 g sample that resulted from the oxidation of glucose and obtain 3.702 g co2 and 1.514 g h2o. how many carbon atoms are in the empirical formula of the isolated sample? (assume no other products present from the oxidation of glucose.)]

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750...
Questions




Chemistry, 22.02.2021 04:50

History, 22.02.2021 04:50


English, 22.02.2021 04:50


Mathematics, 22.02.2021 04:50




Biology, 22.02.2021 04:50

Mathematics, 22.02.2021 04:50


Mathematics, 22.02.2021 04:50




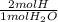 = 0.1682 mol H
= 0.1682 mol H

