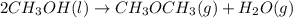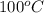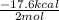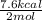Given the standard heat of combustion of methanol ch3oh is 182.6
kcal/mol, dimethyl ether ch3och3 is 347.6 kcal/mol, (methanol/
dimethyl ether in gas phase, water in liquid phase). given the heat
of vaporization of water is 10 kcal/mol, methanol is 8.4 kcal/mol,
dimethyl ether 4.8 kcal/mol. calculate the reaction of dehydration
of methanol to produce dimethyl ether. (indicate the phase of your
components).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1


Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1
You know the right answer?
Given the standard heat of combustion of methanol ch3oh is 182.6
kcal/mol, dimethyl ethe...
kcal/mol, dimethyl ethe...
Questions

Mathematics, 09.11.2020 01:00

English, 09.11.2020 01:00






English, 09.11.2020 01:00

Mathematics, 09.11.2020 01:00


Mathematics, 09.11.2020 01:00

Mathematics, 09.11.2020 01:00



History, 09.11.2020 01:00





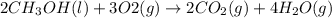 ....... (1)
....... (1)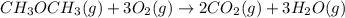 -347.6 kcal/mol
-347.6 kcal/mol
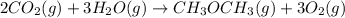 heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)
heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)