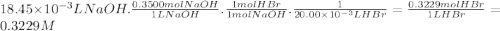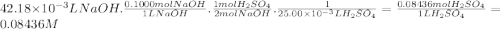Chemistry, 13.11.2019 21:31 ieshaking28
Stu dent has finished his titration, and he comes to you for with the calculations. he tells you that 20.00 ml of unknown concentration hbr(aq) required 18.45 ml of 0.3500 m naoh(aq) to neutralize it, to the point where thymol blue indicator changed from pale yellow to very pale blue. calculate the concentration (molarity) of stu's hbr(aq) sample. kemmi major also does a titration. she measures 25.00 ml of unknown concentration h_2so_4(aq) and titrates it with 0.1000 m naoh(aq). when she has added 42.18 ml of the base, her phenolphthalein indicator turns light pink. what is the concentration (molarity) of kemmi's h_2so_4(aq) sample?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Stu dent has finished his titration, and he comes to you for with the calculations. he tells you th...
Questions

Arts, 04.02.2021 01:10

Mathematics, 04.02.2021 01:10

History, 04.02.2021 01:10

Mathematics, 04.02.2021 01:10


Mathematics, 04.02.2021 01:10

Geography, 04.02.2021 01:10

Mathematics, 04.02.2021 01:10



Mathematics, 04.02.2021 01:10


Social Studies, 04.02.2021 01:10


Mathematics, 04.02.2021 01:10