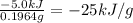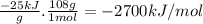Chemistry, 13.11.2019 21:31 joooosshhhh
Quinine is an important type of molecule that is involved in photosynthesis. the transport of electrons mediated by quinone in certain enzymes allows plants to take water, carbon dioxide, and the energy of sunlight to create glucose. a 0.1964-g sample of quinone (c6h4o2) is burned in a bomb calorimeter with a heat capacity of 1.56kj/c. the temperature of the calorimeter increases by 3.2 degrees c. calculate the energy of combustion of quinone per gram and per mole.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Quinine is an important type of molecule that is involved in photosynthesis. the transport of electr...
Questions

Mathematics, 05.10.2020 23:01



Biology, 05.10.2020 23:01






Mathematics, 05.10.2020 23:01


Mathematics, 05.10.2020 23:01

Mathematics, 05.10.2020 23:01


Mathematics, 05.10.2020 23:01

Geography, 05.10.2020 23:01

History, 05.10.2020 23:01



Biology, 05.10.2020 23:01