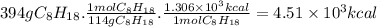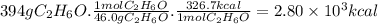Chemistry, 13.11.2019 22:31 shartman22
Ethanol, c2h6o, is most often blended with gasoline - usually as a 10 per cent mix - to create a fuel called gasohol. ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to burn more efficiently in combustion engines. the heat of combustion of ethanol is 326.7 kcal/mol.
the heat of combustion of 2-methylheptane, c8h18, is 1.306×103 kcal/mol. how much energy is released during the complete combustion of 394 grams of 2-methylheptane ?
assuming the same efficiency, would 394 grams of ethanol provide more, less, or the same amount of energy as 394 grams of 2-methylheptane?
a. more or
b. less or
c. the same amount

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:40
Which are causes of mechanical weathering? check all that apply.oacid raino plant growtho animal actionso carbon dioxideo pressure release
Answers: 1

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Ethanol, c2h6o, is most often blended with gasoline - usually as a 10 per cent mix - to create a fue...
Questions

History, 05.04.2020 22:52




History, 05.04.2020 22:52

Mathematics, 05.04.2020 22:52










Chemistry, 05.04.2020 23:44

Mathematics, 06.04.2020 00:03


History, 06.04.2020 00:03

Chemistry, 06.04.2020 00:03